Hydrogen Bonding In Acetic Acid
Hydrogen Bonding
- Page ID
- 1230
The near powerful intermolecular forcefulness influencing neutral (uncharged) molecules is the hydrogen bond. If we compare the boiling points of methane (CH4) -161ºC, ammonia (NH3) -33ºC, water (H2O) 100ºC and hydrogen fluoride (HF) 19ºC, we see a greater variation for these similar sized molecules than expected from the data presented above for polar compounds. This is shown graphically in the post-obit chart. Nigh of the simple hydrides of group 4, V, VI & Vii elements display the expected rising in boiling betoken with molecular mass, but the hydrides of the nigh electronegative elements (nitrogen, oxygen and fluorine) have abnormally high boiling points for their mass.
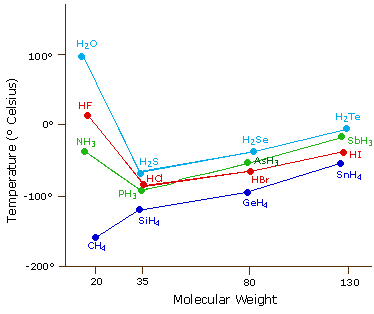
The exceptionally potent dipole-dipole attractions that cause this behavior are called the hydrogen bail. Hydrogen forms polar covalent bonds to more electronegative atoms such every bit oxygen, and considering a hydrogen atom is quite pocket-sized, the positive finish of the bond dipole (the hydrogen) tin arroyo neighboring nucleophilic or bones sites more closely than tin other polar bonds. Coulombic forces are inversely proportional to the sixth power of the distance between dipoles, making these interactions relatively stiff, although they are still weak (ca. 4 to 5 kcal per mole) compared with nearly covalent bonds. The unique properties of water are largely due to the strong hydrogen bonding that occurs between its molecules. In the post-obit diagram the hydrogen bonds are depicted as magenta dashed lines.
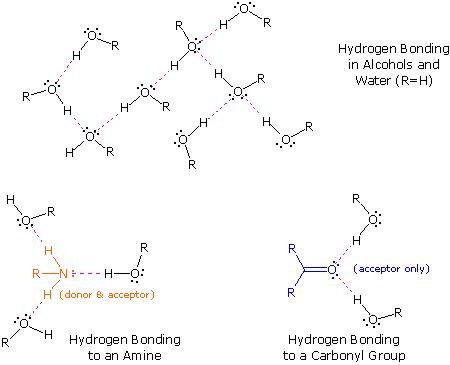
The molecule providing a polar hydrogen for a hydrogen bond is called a donor. The molecule that provides the electron rich site to which the hydrogen is attracted is called an acceptor. Water and alcohols may serve as both donors and acceptors, whereas ethers, aldehydes, ketones and esters can office only every bit acceptors. Similarly, principal and secondary amines are both donors and acceptors, but tertiary amines office simply as acceptors. One time you are able to recognize compounds that tin exhibit intermolecular hydrogen bonding, the relatively high boiling points they exhibit go understandable. The data in the following table serve to illustrate this point.
| Compound | Formula | Mol. Wt. | Boiling Indicate | Melting Indicate |
|---|---|---|---|---|
| dimethyl ether | CHiiiOCH3 | 46 | –24ºC | –138ºC |
| ethanol | CH3CHiiOH | 46 | 78ºC | –130ºC |
| propanol | CH3(CH2)2OH | 60 | 98ºC | –127ºC |
| diethyl ether | (CH3CHtwo)twoO | 74 | 34ºC | –116ºC |
| propyl amine | CH3(CH2)iiNHtwo | 59 | 48ºC | –83ºC |
| methylaminoethane | CHthreeCH2NHCH3 | 59 | 37ºC | |
| trimethylamine | (CHthree)3Northward | 59 | 3ºC | –117ºC |
| ethylene glycol | HOCH2CH2OH | 62 | 197ºC | –13ºC |
| acetic acid | CH3CO2H | sixty | 118ºC | 17ºC |
| ethylene diamine | H2NCHiiCH2NH2 | 60 | 118ºC | 8.5ºC |
Alcohols boil cosiderably higher than comparably sized ethers (start two entries), and isomeric 1º, 2º & 3º-amines, respectively, bear witness decreasing boiling points, with the two hydrogen bonding isomers being substantially higher boiling than the 3º-amine (entries 5 to 7). Likewise, O–H---O hydrogen bonds are clearly stronger than North–H---North hydrogen bonds, as nosotros run into by comparing propanol with the amines.
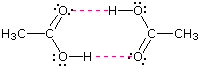
As expected, the presence of ii hydrogen bonding functions in a chemical compound raises the humid point fifty-fifty farther. Acetic acrid (the ninth entry) is an interesting case. A dimeric species, shown on the right, held together by two hydrogen bonds is a major component of the liquid state. If this is an accurate representation of the composition of this chemical compound then we would expect its boiling point to be equivalent to that of a CivH8Ofour compound (formula weight = 120). A suitable approximation of such a chemical compound is found in tetramethoxymethane, (CH3O)4C, which is actually a bit larger (formula weight = 136) and has a boiling betoken of 114ºC. Thus, the dimeric hydrogen bonded structure appears to be a skillful representation of acetic acid in the condensed state.
A related principle is worth noting at this bespeak. Although the hydrogen bond is relatively weak (ca. 4 to five kcal per mole), when several such bonds exist the resulting construction tin can be quite robust. The hydrogen bonds betwixt cellulose fibers confer great forcefulness to wood and related materials. For additional information on this subject Click Here.
Hydrogen Bonding In Acetic Acid,
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_%28Organic_Chemistry%29/Fundamentals/Intermolecular_Forces/Hydrogen_Bonding
Posted by: pettyhattlem88.blogspot.com


0 Response to "Hydrogen Bonding In Acetic Acid"
Post a Comment